Brazil Medical Device Registration Overview
Brazil is one of the biggest countries in South America. It is one of the fastest growing markets for Medical Devices in the world. Brazil spends hugely on healthcare boosting the Medical Devices development locally. The country has established regulations in place, and it governs Medical Devices through National Health Surveillance Agency (ANVISA - Agência Nacional de Vigilância Sanitária) under the Ministry of Health. Foreign manufacturers must appoint a Brazilian Registration Holder (BRH) to assist them with the Brazil medical device registration process.
Regulatory Authority: Agência Nacional de Vigilância Sanitária (ANVISA).
Regulation: RDC 751/2022
Regulatory Pathway: Notification (Notificação) and Registration (Registro)
Authorized Representative: Brazil Registration Holder (BRH) Required
QMS Requirement: Brazilian GMP/MDSAP
Assessment of Technical Data: ANVISA
Labeling Requirements:Chapter VI of RDC 751/2022
Submission Format: Electronic
Language: Brazilian Portuguese/English
Brazil Medical Device Classification
In the ANVISA Regulatory process, the first step in determining the registration path and compliance with Brazilian regulations is to ascertain the classification of the Medical Device. In Brazil, devices are classified into four classes based on risk (Class I-IV).
|
Medical Device Class |
Criteria |
|
Class I |
Low Risk |
|
Class II |
Medium Risk |
|
Class III |
High risk |
|
Class IV |
Maximum Risk |
Brazil Registration Holder (BRH)
Foreign manufacturers who intend to market their Medical Devices in Brazil must appoint a Brazilian Registration Holder (BRH), i.e., an Authorized Local Representative responsible for submitting necessary documents to the ANVISA for registration procedure. A BRH maintains control of the manufacturer’s device registration and BGMP certification, where applicable. BRH is also involved in the coordination with the distributors for the import authorization of each shipment.
ANVISA Medical Device Registration
In Brazil, the device registration is done through Notificação or Registro processes based on the device classification (Class I, II, III and IV) which also decides the extent of scrutiny involved for approval. Further, all the medical device manufacturers need to comply with the Brazilian Good Manufacturing Process (BGMP), which is in line with ISO 13485:2016. The complex, stringent and extensive Regulatory regime makes the process daunting.
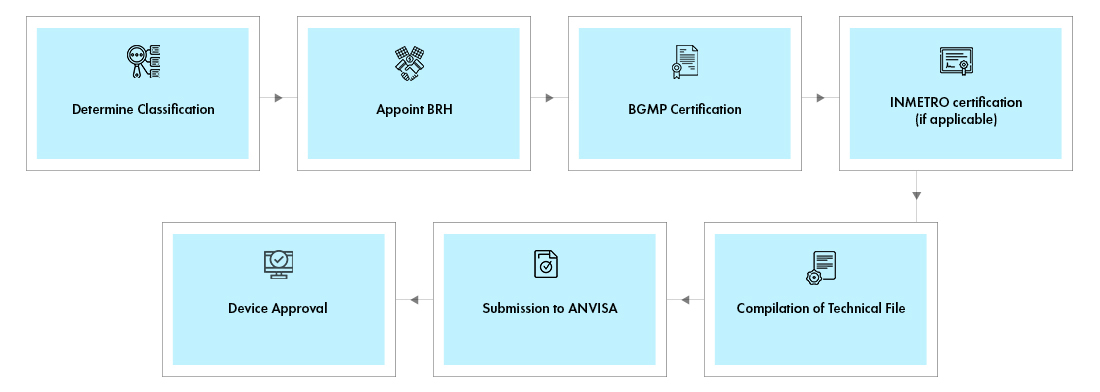
Process flow
Post Approval Services
Freyr supports the foreign manufacturers in end-to-end Medical Device life-cycle Management, including post approval activities, such as –
- Post approval change management - modifications to existing medical device approvals such as, addition of new variants, accessories; addition of new indications of use among others
- Maintenance of approvals and registration through timely payment of administrative and registration fees
- Renewal of licenses
- Liaising between ANVISA and the manufacturer
- Importation Management
Freyr assists manufacturers to identify the Device Classification as a first step. Freyr helps clients in procuring certifications such as, INMETRO and BGMP by helping in rectifying the Regulatory hassles involved. With proven expertise in launching numerous devices in the region, Freyr offers end-to-end Regulatory services for Medical Devices.
Summary
|
Class |
Registration Pathway |
Timelines |
Validity of Registration |
|
Class I Devices |
Notificação |
30 days |
unlimited |
|
Class II Devices |
Notificação |
30 days |
unlimited |
|
Class III Devices |
Registro (Full Registration) |
4 -12 months |
10 years |
|
Class IV Devices |
Registro (Full Registration) |
4 -12 months |
10 years |
Freyr Expertise
- Regulatory due-diligence Services
- Official Classification Services
- BGMP Certification services
- MDSAP certification
- Inmetro Certification
- Economic Information Report (EIR) services
- Device Registration through Notificação and Registro pathways
- Brazilian Registration Holder (BRH) services
- Operating permits and Authorizations
- Translation Services
- Labelling Services
- Distributor Identification and Qualification services
- Post Approval Change Management
- License Renewal and transfer Services
